While no current Food and Drug Administration-approved macular degeneration treatment is likely to completely restore vision lost to macular degeneration, some drugs may be able to preserve or even improve remaining vision. Also, certain investigational treatments have shown promise for reversing at least some vision loss in many AMD patients. [See also: More about macular degeneration.]
FDA-Approved Macular Degeneration Treatments
Lucentis. Approved by the FDA on June 30, 2006 for treating the more advanced or “wet” form of macular degeneration, Lucentis (ranibizumab) is a form of the colorectal cancer treatment drug, Avastin. [See also: Investigational Treatments.] Genentech in collaboration with Novartis Ophthalmics is marketing this new drug, similar in action to Macugen (see below) in that it targets VEGF protein thought to contribute to development of AMD by promoting growth of abnormal blood vessels in the back of the eye (retina).
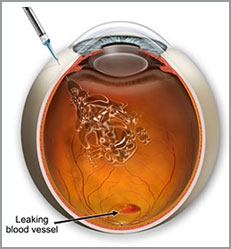
Injections for the “wet” form of macular degeneration are made directly into the eye for treatments including Macugen, Lucentis and Avastin.
Lucentis clinical trial results leading to FDA approval have been extremely promising. In late 2005, Genentech announced results of one study demonstrating improved or stable vision in about 95 percent of participants compared with only about 60 percent of people receiving another treatment. Vision improvement with Lucentis was significant. While only 11 percent of the control group could see 20/40 or better following the study, about 40 percent of Lucentis patients were able to do so. In overall study results, about one-third of patients undergoing clinical trials experienced vision improvement.
Lucentis is administered through monthly injections into the eye. An FDA news release about the approval says rare adverse reactions to the drug mainly were associated with the injection itself. Complications of treatment can include severe inflammation within the eye (endophthalmitis), increased eye pressure (intraocular pressure), traumatic cataract or retinal detachment or tear.
Some eye doctors are debating whether the above-mentioned Avastin should be used in the eye instead of Lucentis. Avastin is significantly less expensive but was not developed as a macular degeneration treatment. For details, read more about the Lucentis vs. Avastin debate.
Macugen. This newer treatment for AMD (pegaptanib sodium) uses a therapeutic molecule to attack a protein that causes abnormal blood vessel growth in the eye; it was FDA-approved in December 2004. [See AMD News.] The drug, developed by Eyetech Pharmaceuticals and Pfizer, is administered through injections into the eye, with treatments required every six weeks. In clinical trials, 33 percent of patients receiving Macugen maintained or improved their vision compared with only 22 percent in the control group. Macugen also helped slow the rate of vision loss for many age related macular degeneration patients.
Moby’s Drug Consult (2006) reports that fewer than 1 percent of patients receiving Macugen experienced serious side effects such as retinal detachments or severe inflammation of interior eye structures (endophthalmitis). Less serious side effects such as eye floaters and discomfort occurred in up to 40 percent of patients.
Visudyne drug treatment (Photodynamic Therapy or PDT). Visudyne was the first drug therapy for treatment of the wet form of the disease. It is only for those patients who have new blood vessel growth (neovascularization) under the retina in a well defined, distinctive pattern known as “predominantly classic.” About 40-60 percent of new wet AMD patients have this form of macular degeneration, according to Novartis, the company that markets Visudyne (QLT Inc. developed it).
In this treatment procedure, the doctor injects Visudyne into your arm, then activates the drug as it passes through the retinal blood vessels by shining a non-thermal laser with a specific wavelength into your eye. Visudyne is activated by the laser light, which produces a chemical reaction that destroys abnormal blood vessels. The procedure is virtually painless, according to Novartis.
AREDS Nutritional Formula For Macular Degeneration
Research suggests that antioxidant vitamins, such as beta-carotene (vitamin A) and vitamins C and E, may protect the macula from damage.
AREDS study results (National Eye Institute) released in 2001, involving more than 3,600 people, found that supplementation with vitamins C and E, beta-carotene and zinc reduced certain patients’ risk of progressing to advanced AMD by about 28 percent. This number reflects those patients with large numbers of intermediate or large yellowish deposits (drusen) on their retinas, but not those with limited intermediate drusen or multiple small drusen.
Because of their findings, the researchers recommend that patients at risk of developing advanced AMD consider taking antioxidant and zinc supplements. Participants received:
- 500 milligrams (mg) of vitamin C
- 400 international units (IU) of vitamin E
- 15 mg of beta-carotene
- and 80 mg of zinc oxide
The eye care community does not agree on the benefits of zinc or antioxidant supplements: more study is needed, especially on the long-term effects of high-dose supplementation. High doses of beta carotene have been associated with an increased risk of cancer in smokers. Keep in mind also that too much of any vitamin or mineral may affect the body’s ability to absorb other important nutrients, so follow your doctor’s advice about dosage.
Read more about how vitamins and other nutrients can affect the eyes. Also learn how you may volunteer to participate in the AREDS II study sponsored by the National Eye Institute. – M.H.
One in six Visudyne patients shows improved vision, or about twice as many patients as those who do not undergo Visudyne therapy. Recent studies also indicate significant slowing of AMD progression in many patients using Visudyne drug treatment. The reference book Ophthalmology (Mosby, 2004) reported positive results of one study in which 225 eyes received Visudyne drug treatment, compared with 114 eyes that did not. After 24 months, legal blindness occurred in 28 percent of the Visudyne group and in 45 percent of the non-treated group.
Other light-activated drugs that operate in ways similar to Visudyne also are under investigation.
Laser treatment. Laser photocoagulation can help wet AMD patients by destroying or sealing new blood vessels to prevent leakage, but no longer is widely used as a treatment. The procedure can produce scars, which are perceived as blind spots by the patient. Scientists are working on ways to reduce the scarring and are also studying laser treatments for dry macular degeneration, but progress has been slow.
The reference book Ophthalmology (Mosby, 2004) says only about 15-20 percent of patients with the wet form of AMD have the type of extremely distinctive bleeding under the retina (choroidal neovascularization or CNV) that would qualify them for this type of treatment. Also, the reference book notes that photodynamic therapy (PDT) using Visudyne drug treatment [see above] has all but replaced laser photocoagulation as the preferred treatment for this type of macular degeneration patient.
Please click here to read about investigational macular degeneration treatments that are not yet FDA-approved.

